HOME > Research Summaries > Analysis of Tumor Suppressor Gene using Molecular Imaging for Personalized Medicine
Research Summaries

Analysis of Tumor Suppressor Gene using Molecular Imaging for Personalized Medicine
Natsuko Chiba
Associate Professor
Department of Molecular Immunology Institute of Development, Aging and Cancer
E-mail:![]()
Abstract
BRCA1 is the first susceptibility gene linked to breast and ovarian cancer. To elucidate the molecular function of BRCA1 as a tumor suppressor, we have been analyzed the various functions of BRCA1 including DNA repair. Recently, we are analyzing the cellular response of BRCA1 to various DNA damage using molecular imaging technique. Real-time analysis of DNA repair processes by laser micro-irradiation in living cells demonstrated that different interaction of BRCA1 with repair factors at damaged sites influence its multifunctional roles in DNA repair. Lower expression or function of BRCA1 is also observed in sporadic cancers. Precise analysis of BRCA1-related protein will contribute to the development of novel molecular diagnosis and therapies for personalized medicine.
1. Intoroduction
Our research has been focused on the breast-cancer susceptibility gene 1, BRCA1. The risk of breast cancer developing is about 80% and the risk of ovarian cancer is about 40% in carrier of BRCA1 mutations. BRCA1-related breast cancers are characterized by early onset, a high frequency of bilateral involvement, and poor prognosis.
Somatic BRCA1 mutations are rarely observed in sporadic breast cancer. However, both BRCA1 mRNA and protein expression are down regulated in approximately 30% of sporadic breast cancer and 70% of ovarian cancers. It is believed that this may be due to nonmuational mechanism such as acquired methylation of the promotor or malfunctions in the upstream pathway that regulate BRCA1 expression. Therefore, lower expression or function of BRCA1 is thought be an important contributing factor in sporadic cancers.
BRCA1 has been implicated in a number of cellular processes, including the regulation of DNA damage repair, transcription, cell cycle, chromatin remodeling, and apoptosis (Fig. 1). Among these function, the tumor suppressor activity of BRCA1 is though to be primarily attributed to its involvement in DNA repair. However, the precise mechanism of what role BRCA1 plays in DNA repair pathway is not fully understood.
2. Results
To elucidate the molecular mechanisms of tumor suppressive function of BRCA1, we have been analyzed the various functions of BRCA1. Using biochemical technique, we resolved four distinct BRCA1-containing complexes. BRCA1 content of these complexes is altered following DNA damage and that one of these complexes, the HUIC (hydroxyurea induced complex), is associated with DNA damage. These suggest that BRCA1 participates in multiple cellular prosesses by forming multiple protein complexes [1]. Furthermore, we found that BARD1, heterodimer partner of BRCA1, is an integral component of RNA polymerase II holoenzyme (holo-pol) and that the amino-terminus of BRCA1 is important for the association with the holo-pol and the localization at nuclear foci in the S-phase of cell cycle [2]. Expression of the amino terminus deleted BRCA1 revealed a powerful growth suppressive effect. Its growth suppression is associated with an increase in apoptosis [3]. In addition, we identified RNA polymerase II (Pol II) as a substrate of ubiquitnation activity of BRCA1. Hyperphosphorylated form of Pol II is recognized by the carboxy terminus of BRCA1 as a substrate after UV irradiation, suggesting that BRCA1 is involved in transcription coupled repair process [4]. Furthermore, we developed the functional assay for the molecular diagnosis of BRCA1 by ubiquitination activity and subcellular localization in S-phase and after DNA damage.
Recently, our research has been focused on the DNA repair function of BRCA1. We collaborate with Dr. Akira Yasui, Department of Molecular Genetics, Institute of Development, Aging and cancer, Tohoku University, and are analyzing the cellular response of BRCA1 to various DNA damage using molecular imaging technique. To elucidate the molecular mechanisms of DNA repair function of BRCA1, we are analyzing the BRCA1 accumulation at the DNA damaged site induced by laser micro-irradiation in living cells.
Our experimental system is a laser micro-irradiation apparatus combined with a confocal microscopy. We use a 365nm pulse laser system for the irradiation of cells in the epi-fluorescence path of the microscope system. A lower dose or higher dose of irradiation was obtained by passing lasers through distinct filters in front of the lens. A 405nm pulse laser system is also used. The power of laser scan can be controlled by the number of scan. By using these system, various types of DNA damage, such as single-strand breaks (SSBs), double-strand breaks (DSBs) and oxidative base damage, were produced at restricted nuclear regions of cells [5, 6] (Fig. 1, 2).
Using this system, we found that different interaction of BRCA1 with repair factors at DSBs influence its multifunctional roles in DNA repair.
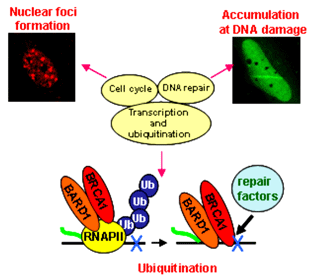
Fig.1. BRCA1 participates in the multiple functions.
Furthermore, we are trying to identify new BRCA1 associated proteins by proteomic study and examining the property of DNA repair pathway and the effect to drug sensitivity by inhibition with RNA interference.
Recently, it is reported that inhibition of poly(ADP-ribose) polymerase 1 (PARP1), which is a critical enzyme to SSB repair pathway, leads to severe, highly selective toxicity in BRCA1-deficient cells [7]. This seems to be because the inhibition of PARP results in unrepaired SSBs, giving rise to DSBs. So, these represent a new concept in cancer treatment. Lower expression or function of BRCA1 is also observed in sporadic cancers. Therefore, they are suggesting thatprecise analysis of BRCA1-related protein will contribute to the discovery of novel molecular target of cancer chemotherapy.
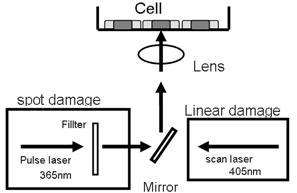
Fig.2. Laser irradiation system
3. Conclusion
In cancer treatment, it is important to select the treatment method and predict its effectiveness in individual cancer patients. Molecular targeting therapies are focuses of investigation with a view to developing novel approach to cancer control. New biomarkers are similarly viewed as essential for improvement of cancer diagnostics. Our research is focused on identification and functional analysis of the critical proteins that play a key role in the carcinogenesis, and development of the biomarker of diagnosis and molecular targets for therapies for personalized medicine. Molecular imaging technique will be a useful tool for those aims.
References
[1] Chiba N, and Parvin, J D. Redistribution of BRCA1 among four different protein complexes following replication blockage. J Biol Chem 276, 38549-38554, 2001.
[2] Chiba N and Parvin, J D. The BRCA1 and BARD1 association with the RNA polymerase II holoenzyme. Cancer Res 62, 4222-4228, 2002.
[3] You F, Chiba, N, Ishioka C, and Parvin, J D. Expresssion of an amino-terminal BRCA1 deletion mutant causes a dominant growth inhibition in MCF10A cells. Oncogene 23, 5792-5798, 2004.
[4] Starita L.M, Howitz A. A, Keogh M-C, Ishioka C, Parvin, J D, and Chiba N. BRCA1/BARD1 ubiquitinate phosphorylated RNA polymerase II. J Biol Chem 280, 24498-24505, 2005.
[5] Lan L, Nakajima S, Oohata Y, Takao M, Okano S, Masutani M, Wilson SH, Yasui A. In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc.Natl.Acad.Sci.USA 101, 13738-13743, 2004.
[6] Lan L, Nakajima S, Komatsu K, Nussenzweig A, Shimamoto A, Oshima J, Yasui A. Accumulation of Werner protein at DNA double-strand breaks in human cells.J Cell Sci 118, 4153-4162, 2005.
[7] Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917-921, 2005.