HOME > Research Summaries > Non-Invasive Treatment with Focused Ultrasound Enhanced with Nano- to Micro-Particles
Research Summaries

Non-Invasive Treatment with Focused Ultrasound Enhanced with Nano- to Micro-Particles
Shin-ichiro Umemura
Department of Biomedical Engineering, Graduate School of Biomedical Engineering
E-mail:![]()
Abstract
The bioeffect of ultrasound can be enhanced by orders of magnitude with microbubbles. Enhancement of ultrasonic tissue absorption with a microbubble agent is demonstrated both in theory and in a vivo experiment. This effect will be useful in therapeutic application of ultrasound if a microbubble agent is delivered selectively to the target tissue.
1. Introduction
Ultrasound has moderate tissue attenuation and absorption coefficients at an appropriate wavelength for penetrating intervening tissues to reach, vibrate, and heat non-superficial tissue objects while maintaining the ability to focus energy into small volumes. This is a unique advantage when compared to electromagnetic modalities such as laser beams in the application to non-invasive treatment of non-superficial tumors. Bioeffects of ultrasound, which can be potentially be used for such a kind of treatment, are shown in Fig. 1.
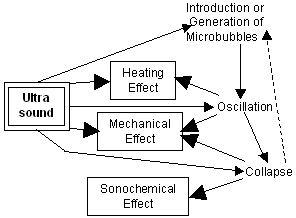
Fig. 1. Bioeffects of ultrasound and their enhancement with microbubbles.
In the existence of microbubbles, the ultrasonic vibration amplitude is enhanced in their vicinity by orders of magnitude, and both mechanical and heating effects can be thereby accelerated. Furthermore, sonochemical effects can be induced when microbubbles collapse.
Recently, the heating effect was started being clinically used as the thermal coagulation treatment with high intensity focused ultrasound (HIFU). In this paper, the acceleration of heating [1] is chosen as an example of the ultrasonic bioeffects enhanced with microbubbles.
The ultrasonic power absorbed by a microbubble per volume is theoretically predicted and plotted in Fig. 2 against the microbubble radius with the ultrasonic intensity as a parameter. It is estimated that the number of resonant microbubbles in the order of 10 per mm3 can double the ultrasonic absorption of tissue.
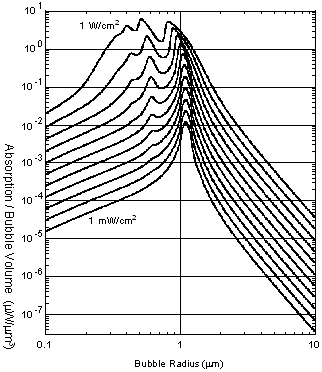
Fig. 2. Ultrasonic abosorption by microbubble at 3 MHz, theoretically predicted by numerically solving the Rayleigh-Plesset equation. Ultrasonic intensity is varied as a parameter from 1 mW/cm2 to 1 W/cm2 in a geometric series.
2. Methods
Animal experiments were performed using a HIFU transducer at 3 MHz with a focal length of 30 mm. A schlieren image of the focal field is shown in Fig. 3. An exteriorized kidney of an anesthetized rat was exposed to HIFU in degassed saline as shown in Fig. 4. A very thin thermocouple was inserted into in the kidney cortex at the ultrasonic focal point. A microbubble agent, Optizon®, was injected into the rat through a jugular vein at a dose of 0.2 ml/kg. This is an order of magnitude higher than normal clinical dose, but still within the safe dose range. A relatively low HIFU intensity was used so that thermal coagulation of tissue should not be induced with ultrasound alone.
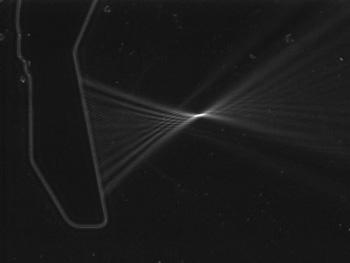
Fig. 3. Focal ultrasonic field at 3 MHz.
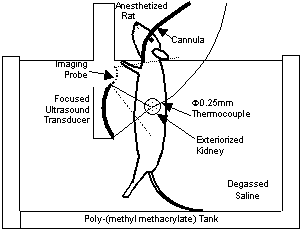
Fig. 4. Experimental setup of HIFU exposure.
3. Result
The tissue temperature elevation due to HIFU exposure with and without Optison is shown in Fig. 5.
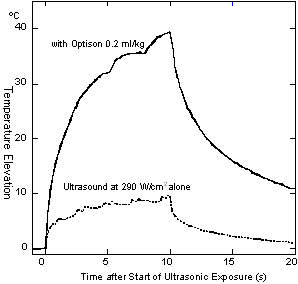
Fig. 5. Tissue temperature elevation with HIFU.
4. Conclusion and Future Work
It was demonstrated that a microbubble agent multiplies the heating effect of ultrasound. However, if it is distributed in the whole body at a similar tissue concentration, the ultrasonic attenuation in the intervening tissue will be multiplied at the same time. The obtained gain with the microbubble injection will be mostly cancelled with the attenuation. A microbubble agent should be delivered selectively to the target tissue to fully utilize the observed effect.
The delivery of microbubbles to target tissue as phase conversion nano-particles may have the potential to satisfy this requirement. A liquid particle approximately 100 nm in radius, which may have the EPR effect to accumulate in tumor tissues, can be converted with ultrasonic stimulation to a gas bubble approximately 1 μm in radius, which can efficiently absorb ultrasound in a MHz range [2].
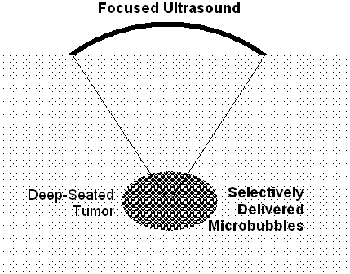
Fig. 6. Selective delivery of microbubbles.
Acknowledgements
The author acknowledges Kazuaki Sasaki and Ken-ichi Kawabata of Hitachi Central Research Laboratory for collaboration in producing the experimental result.
References
[1] Umemura S, Kawabata K, and Sasaki K. Acceleration of ultrasonic tissue heating by microbubble agent. IEEE Trans. Ultrasonics, Ferroelectrics, and Frequency Control 52, 1690-1698, 2005.
[2] Kawabata K, Sugita N, Yoshikawa H, Azuma T, and Umemura S. Nanoparticles with Multiple Perfluorocarbons for Controllable Ultrasonically Induced Phase Shifting. Jpn. J. Appl. Phys., 44(6B), 4448-4852, 2005.