HOME > Research Summaries > Inner Ear Biomechanics
Research Summaries

Inner Ear Biomechanics
Hiroshi Wada
Professor
Department of Bioengineering and Robotics, Graduate School of Engineering
E-mail:![]()
1. Introduction
Figure 1 shows a schematic of the human auditory system. Sound enters the external ear canal and vibrates the tympanic membrane. The mechanical vibration of the tympanic membrane is then transmitted to the cochlea via the ossicles. The cochlea is a spiral-shaped organ, which is filled with lymph fluid. The organ of Corti, containing inner hair cells (IHCs), outer hair cells (OHCs) and various kinds of supporting cells, sits on the basilar membrane in the cochlea and is covered by the tectorial membrane.
The vibration of the tympanic memrane and the ossicular chain, which is induced by sound, is transmitted to the basilar membrane and the organ of Cotri through the cochlear fluid. IHCs transform the vibration of the organ of Corti into action potentials in the auditory nerve fibers, this process being known as mechano-electrical transduction. The resulting signals are transmitted to the brain. As a result, we can finally perceive the sound. Simultaneously, OHCs alter their length in response to acoustic stimuli, which is known as electromotility. The force generated by this electromotility is thought to amplify the vibration of the organ of Corti, thus realizing the high sensitivity, wide dynamic range and sharp tuning of our auditory system. The electromotility of OHCs is believed to be driven by conformational changes of the motor protein "prestin," which is distributed in the OHC lateral wall membrane. Prestin is considered to be a high-performance motor protein with characteristics of a piezoelectric element.
Our research themes are as follows: (1) structural analysis of the motor protein prestin and development of a bio-microactuator driven by prestin, (2) development of new treatments for hereditary deafness caused by mutations in membrane proteins expressed in the inner ear, (3) numerical analysis of the dynamic behavior of the organ of Corti by the finite element method (FEM).
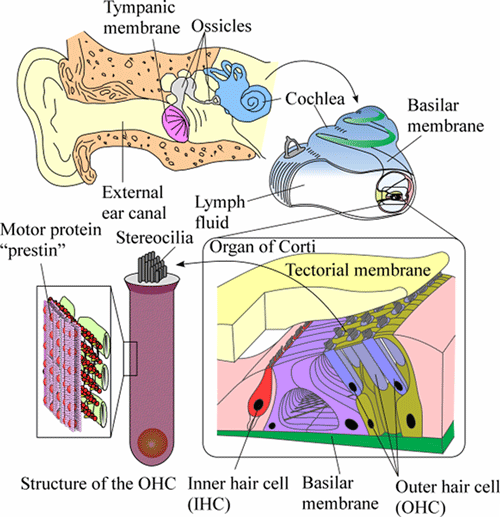
Fig. 1. Schematic of the human auditory system.
2. Study on the Motor Protein Prestin
2.1. Structural analysis of prestin
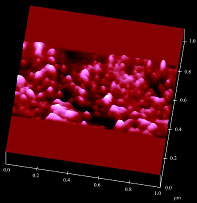
Fig. 2. Atomic force microscope image of a portion
of the plasma membrane of a prestin-transfected cell.
We have attempted to determine the structure of prestin, which consists of 744 amino acids and is a member of the solute carrier (SLC) 26A family. Figure 2 shows an atomic force microscope image of a portion of the plasma membrane of a prestin-transfected cell. Particle-like structures ranging in diameter of from several nanometers to several tens of nanometers were observed. Analysis of the size of the observed structures revealed that the diameter of prestin was 8-12 nm [1]. In a further study, we will attempt to determine the three-dimensional structure of prestin using purified proteins by high-resolution atomic force microscopy.
2.2. Development of a bio-microactuator driven by prestin
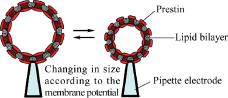
Fig. 3. Voltage-driven lipid vesicles using prestin.
It is known that OHC somatic motility is completely synchronized by electrical stimuli up to 100 kHz. The efficiency of OHC conversion of electrical stimulation to mechanical displacement is very high, the voltage sensitivity of 20 nm/mV for a 50-μm-long OHC being four orders of magnitude greater than the best man-made material. In addition, prestin is considered to be a direct voltage-force converter which is independent of ATP. We aim to develop a new type of bio-microactuator driven by the high-performance motor protein prestin. To achieve this, we will attempt to develop voltage-driven lipid vesicles by reconstitution of purified prestin into lipid bilayers (Fig. 3). Employment of these vesicles as biocompatible actuators is envisioned.
3. Development of New Treatments for Hereditary Deafness by Rescue of Membrane Protein Mutants
Deafness is one of the most common congenital diseases, severe hearing loss occurring in one per 1,000 neonates. Congenital hearing loss is believed to be related to gene mutations in more than 50% of such cases. Some of the mutations in membrane proteins expressed in the inner ear, such as connexin 26, which is a component of gap junctions, and pendrin, which is a transporter of chloride and iodide, are considered to lead to dysfunction of the proteins due to misfolding, resulting in deafness.
We have shown that by adding a reagent which may act as a chaperone, the function of misfolded mutants of prestin [2], which is a homologue of pendrin, can be recovered, i.e., disrupted structures of prestin mutants can be corrected. Based on this finding, we will attempt to develop new treatments for hereditary deafness by adding reagents acting as chaperones, which may facilitate correct refolding of the misfolded membrane proteins.
4. Numerical Analysis of the Dynamic Behavior of the Organ of Corti by the Finite Element Method
To clarify the auditory transduction process, it is important to investigate the dynamic behavior of the organ of Corti. However, due to the vulnerability of the cochlea and the tiny displacement amplitude of the organ of Corti vibration, observation of the vibration pattern of the organ of Corti is difficult. In this study, the dynamic behavior of the organ of Corti was therefore analyzed by newly developed FEM software. Using a finite-element model of the gerbil organ of Corti (Fig. 4), it was found that the inner structure of the organ of Corti remains unchanged and vibrates as one rigid body during the vibration. The frequency characteristics of the phases of the displacement of the hair bundle and the neural excitation relative to the displacement of the organ of Corti were also clarified [3].
In a further study, we will attempt to analyze the effect of OHC motility on the vibration pattern of the organ of Corti by introduction of such motility into the model. We aim to reveal the sound reception mechanism and the effect of lesions on such mechanism.
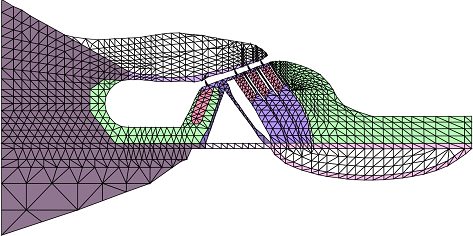
Fig. 4. Finite element model of the organ of Corti.
References
[1] Murakoshi M, Gomi T, Iida K, Kumano S, Tsumoto K, Kumagai I, Kobayashi T, and Wada H. Imaging by atomic force microscopy of the plasma membrane of prestin-transfected Chinese hamster ovary cells. J Assoc Res Otolaryngol 7, 267-278, 2006.
[2] Kumano S, Iida K, Murakoshi M, Naito N, Tsumoto K, Kobayashi T, Ikeda K, Kumagai I, Kobayashi T, and Wada H. Effects of mutation in the conserved GTSRH sequence of the motor protein prestin on its characteristics. JSME Int J 48C, 403-410, 2005.
[3] Andoh M, Nakajima C, and Wada H. Phase of neural excitation relative to basilar membrane motion in the organ of corti: Theoretical considerations. J Acoust Soc Am 118, 1554-1565, 2005.