HOME > Research Summaries > Biomechanical Study of Cell Mechanosensing
Research Summaries

Biomechanical Study of Cell Mechanosensing
Masaaki Sato
Professor
Department of Biomedical Engineering, Graduate School of Biomedical Engineering
E-mail:![]()
1. Introduction
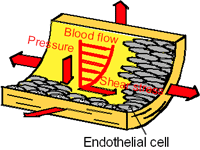
Fig. 1. Vascular endothelial cells exposed
to various mechanical forces
Most living cells have an ability to sense the direction and magnitude of external mechanical stimuli as mechanosensing system. This ability is called a "mechanosensor". For example, vascular endothelial cells (ECs) lining vessel lumens are constantly exposed to fluid shear stress due to blood flow, stretch due to wall deformation, and hydrostatic pressure due to blood pressure (Fig. 1), and respond to their mechanical environment showing alterations in cell shape and physiological functions [1]. However, little is known of how cells can sense mechanical stimuli leading to activation of intracellular signaling cascades.
So far, many studies relating to cell responses to mechanical stimuli have been done to investigate the mechanism of mechanosensing from a viewpoint of molecular biology or biochemistry. These approaches should not be enough to explain the applied force-dependent cell responses, for example, ECs elongate and align in the direction of flow. In our laboratory, we have exclusively studied morphological responses of ECs exposed to fluid shear stress and have revealed following features of cell adaptation process in morphology: (1) disassembly of stress fibers, (2) development of lamellipodia, (3) rearrangement of focal adhesions, and (4) formation of stress fibers. We have also found that expression of VE-cadherin in the intercellular junctions may be closely associated with alterations in cell morphology (Fig. 2). From theses results, we have speculated that stress fibers, intercellular junctions, and focal adhesions can be candidates for mechanosensors and may play crucial roles in determination of cell morphology under mechanical conditions.
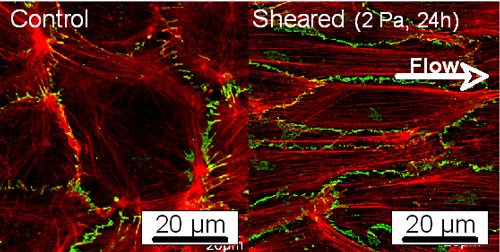
Fig. 2. Fluorescent images of actin filaments (red) and VE-cadherin (green) before and after exposure to shear stress
In this study, we hypothesize that stress fibers, intercellular junctions, and/or focal adhesions may be involved in the mechanism of mechanosensing. To test this hypothesis, we apply mechanical stimuli including shear stress, tensile stress, and hydrostatic pressure to ECs and identify the candidate for mechanosensor by combining live-cell imaging, micro-mechanical tests, and bio-MEMS techniques.
2. Research Projects
2.1. Live-cell imaging of ECs exposed to mechanical stimuli
In order to observe the dynamic behavior of focal adhesions, VE-cadherin, actin filaments, and their interactions, gene transfection techniques with red fluorescent protein-tagged focal adhesion targeting (FAT) and green fluorescent protein (GFP)-tagged actin are introduced. FAT domain is the amino acid sequence at the C-teminus of focal adhesion kinase. For VE-cadherin imaging, we have newly constructed a yellow fluorescent protein-VE-cadherin vector. Rearrangement of microtubules is also observed because they are supposed to be associated with formation of focal adhesions. In addition to dynamic behavior of these proteins, we are planning to focus on activities of Rac1, which is one of the Rho family of GTPases and known to regulate development of lamellipodia and affect formations of both focal adhesions and intercellular adhesions.
2.2. Application of local stretch at intercellular junctions
To particularly focus on roles of intercellular junctions involved in the mechanism of mechanosensing, we utilize a micropipette technique to apply local mechanical stimuli to ECs. After applying local stretch to an EC expressing GFP-actin, changes in morphology and stress fiber structures in the neighboring cells induced by tension transmitted through the intercellular junctions are examined (Fig. 3). We also observe localization of SHP-2, which is a tyrosine phosphatase and believed to be associated with signaling pathway of stress fiber formation.
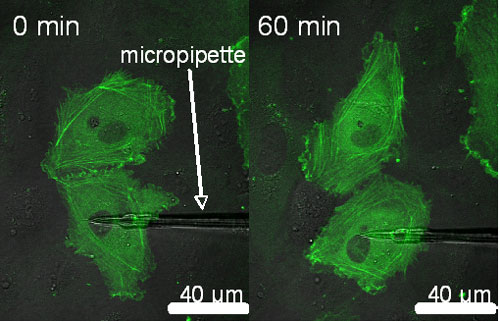
Fig. 3. Fluorescent images of GFP-actin before and after exposure to local stretch
2.3. Micro-tensile test of stress fibers
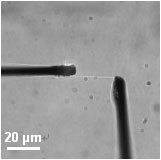
Fig. 4. Tensile test of an isolated single stress fiber
Since assembly and disassembly of stress fibers have been observed in ECs exposed to mechanical stimuli [2], it can be speculated that stress fibers may act as a mechanosensor, possibly recognizing the direction and magnitude of mechanical stimuli. However, how mechanical forces contribute to disassembly of stress fibers is still unclear. We have previously developed a tensile tester for single stress fibers isolated from cultured smooth muscle cells (Fig. 4) [3,4]. Using the tensile tester, we examine the relationship between tensile forces and disassembly of stress fibers, focusing on roles of cofilins in disassembly of stress fibers.
2.4. Development of micro-fluidic system using Bio-MEMS technique
Conventional studies to know the effect of mechanical stimuli on cell responses include a system, which apply global stresses to a large number of cells. This technique, however, is inadequate for studying individual mechanosensors due to the difference in scale. To overcome this difficulty, it is required to develop a micro-fluidic system, which can quantitatively control micro-mechanical environment surrounding a cell. By combining the micro-fluidic system and live-cell imaging techniques, we will establish a novel experimental system for the mechanism of mechanosensing.
3. Summary
In this study, we investigate the detailed mechanism of cell mechanosensing, with observation of dynamic behavior of intracellular structures using live-cell imaging, measurements of mechanical properties of cell organelles, and controls of micro-mechanical environment using Bio-MEMS technique. In the series of this study, the following topics will be particularly focused: (1) observation of cell shape and cytoskeletal structures, (2) observation of intercellular junction-associated proteins, focal adhesion-associated proteins, and signaling proteins at molecular levels, (3) measurements of mechanical properties of organelles, and (4) quantification of intracellular stress states. The point of this study is to quantify cellular responses to mechanical stimuli, from the viewpoint of not only molecular biology but also cell biomechanics. This multi-technique approach would further promote to study the mechanism of cell mechanosensing.
References
[1]Davies P. Flow-mediated endothelial mechano-transduction. Physiol Rev 75, 519-560, 1995.
[2] McCue S, Noria S, and Langille BL. Shear-induced reorganization of endothelial cell cytoskeleton and adhesion complexes. Trends Cardiovasc Med 14, 143-151, 2004.
[3]Deguchi S, Ohashi T, and Sato M. Evaluation of tension in actin bundle of endothelial cells based on preexisting strain and tensile properties. Mol Cell Biomech 2, 125-134, 2005.
[4] Deguchi S, Ohashi T, and Sato M. Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J Biomech 39, 2603-2610, 2006.